(Des Moines, Iowa) – COVID-19 rates are rising again in Iowa, according to new state and federal data, as the U.S. Food and Drug Administration approved emergency use of new COVID-19 vaccines in an effort to combat rising spread of coronavirus variants.
The Iowa Capital Dispatch reports, according to new Iowa Department of Health and Human Services data, positive COVID-19 tests grew to 21.9% in the week of Aug. 18 through 24, a 0.1% increase from the previous week, but is still lower than the 22.7% positive rate earlier in August. However, the positive testing rate is sizably higher than the 16.8% reported during the week of Aug. 26 in 2023.
Iowa, alongside many other states are seeing rising cases of COVID-19 due to “FLiRT variants,” researchers say, a category of several strains of COVID-19 that have mutated in similar ways. “FLiRT” infections are associated with milder symptoms than previous COVID strains, but still pose a risk to vulnerable populations such as immunocompromised and elderly individuals. The state department also reported a 3% rate of emergency room visits and 2.7% rate for inpatient visits that were related to COVID-19.
There were two Iowans who died from COVID-19 in the week period, HHS data found, lower than the six deaths reported in the same period in 2023.
At the same time, Iowa also saw a rise in COVID-19 wastewater viral activity. Data from The U.S. Centers for Disease Control found that Iowa has “very high” viral wastewater activity for the week ending Aug. 24, measured from nine state wastewater sites reporting on coronavirus rates detectable in water. This system allows health officials to monitor the spread outside of hospital visits and COVID tests, according to the CDC, in addition to being able to detect cases in which infected people are asymptomatic.
The current rating is an increase from the “high” viral wastewater activity reported in Iowa earlier this month. Iowa’s Wastewater Viral Activity Level was measured at 9.77 in the most recent data reported, a decrease from the 11.39 level measured for the week ending Aug. 17, but still above the national 8.78 national rating for the same period. Any score above eight points is considered a “very high” activity level.
The FDA last week granted emergency use authorization for the 2024-2025 coronavirus vaccines, an updated version of the Novavax COVID-19 vaccine that administration officials said more closely targets variants that are currently spreading. Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said the authorization allows Americans access to “an additional COVID-19 vaccine option that meets the FDA’s standards for safety, effectiveness and manufacturing quality needed.”
“The COVID-19 vaccines have had a tremendous positive impact on public health and vaccination continues to be the most effective method for COVID-19 prevention,” Marks said in a statement. “COVID-19 continues to be a very real risk for many people, and we encourage individuals to consider getting an updated COVID-19 vaccine when eligible.”
People age 12 and older who have never received a COVID-19 vaccination are eligible to receive two doses, three weeks apart, of the updated vaccine. Those who have already received a form of Novavax COVID-19 vaccine, or been vaccinated with using a different formula from another manufacturer, are eligible to receive doses of the updated vaccine within specified time frames since their previous vaccination.
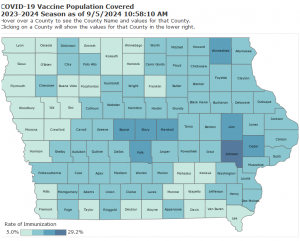
https://data.idph.state.ia.us/t/IDPH-DataViz/views/COVID-19VaccineImmunization/COVID-19Vaccine?%3Aembed=y&%3AisGuestRedirectFromVizportal=y
Iowa has an immunization rate of 15.7% statewide. Public health officials recommend that Iowans stay up to date on vaccinations, and that people age 5 and older get one dose of the updated vaccine to prevent serious illnesses.









